

The alkaline earth metals, among which is magnesium, have properties such as being soft, colored and having a low density. Elements like magnesium have low ionization energy. All alkaline earth metals form ionic compounds except for beryllium. You will be able to see magnesium qualities such as its melting and boiling point, its magnetic properties or what its chemical symbol is. In addition, here you will find information about its atomic properties such as the distribution of electrons in magnesium atoms and other properties.įor some elements, some of this information is unknown. In these cases we show the properties attributed to them. On this page you can discover the chemical properties of magnesium and information about magnesium and other elements on the periodic table such as beryllium, calcium, sodium or aluminum. You will also learn what magnesium is for and you will know what its uses are through its properties associated with magnesium such as its atomic number or the usual state in which magnesium can be found. You Can Visit Our Managed: Periodic Table Main Page Its symbol is Mg and belongs to the group of alkaline earth metals and its usual state in nature is solid. Magnesium is located at position 12 on the periodic table.
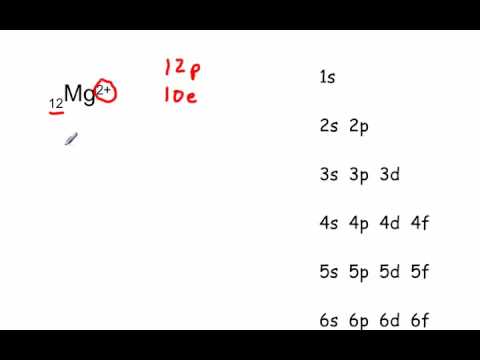
Magnesium is a silver-white chemical element with an atomic number of 12. We elaborate the uses of magnesium and atomic properties with characteristics.


 0 kommentar(er)
0 kommentar(er)
